Facilitate medical research that advances human health

 1-703-853-8036
1-703-853-8036

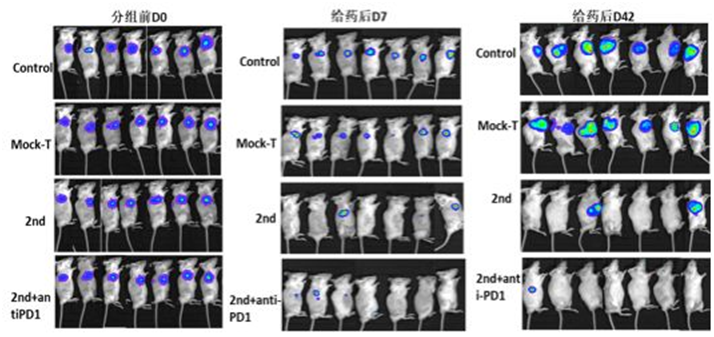
Figure 1 PerkinElmer’s IVIS Lumia III Bio-Real in vivo imaging equipments
SAFE Pharmaceutical Research Institute-PerkinElmer Collaborative Innovation Center of New Drug Evaluation
SAFE Pharmaceutical - PerkinElmer New Drug Evaluation Collaborative Innovation Center, owns international advanced equipment such as IVIS Lumina III imaging system. By bioluminescence or fluorescent labeling, cells, proteins, antibodies, nucleic acids and other drug molecules can be labeled, and the highly sensitive IVIS Lumina III system can be used to study the biological response and targeted distribution of the subject in vivo. In the non-invasive situation, real-time observation of tumor growth and metastasis in animals, distribution and metabolism of materials or drugs in the body, gene expression and other biological processes have been widely used in basic research and drug research and development. At present, the Collaborative Innovation Center has established a one-stop pre-clinical evaluation technology platform for anti-tumor drugs, CAR-T therapy, stem cell therapy, gene therapy drugs and immunotherapeutics. In the majority of scientific research personnel and drug research and development companies to provide high quality and efficient services.
Tel
Mr. Du(18800003391)
E-mail:dujie@safeglp.com
Mr. Zhang(17801093678)
E-mail:zhangxiaolei@safeglp.com
