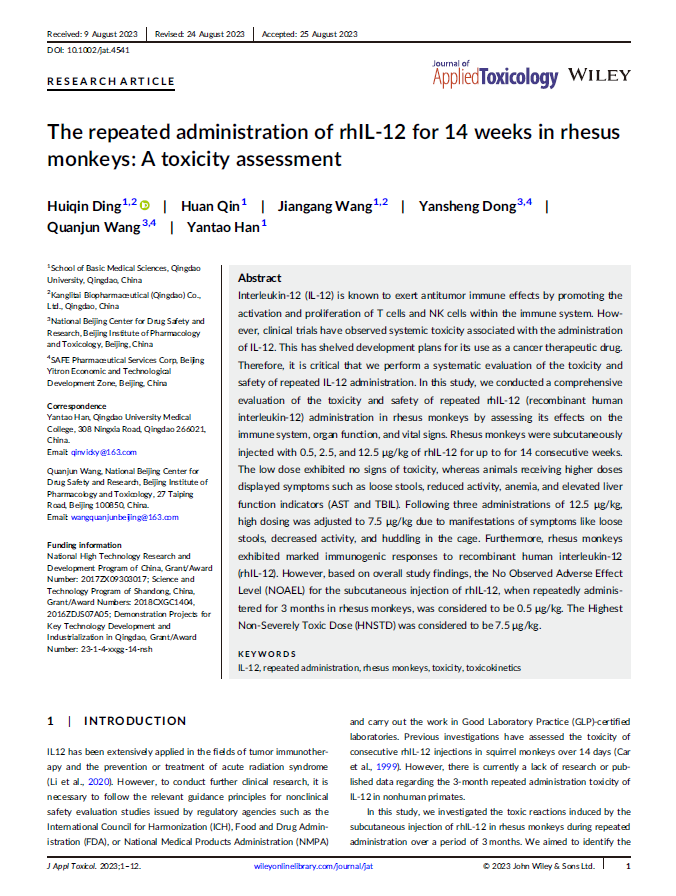
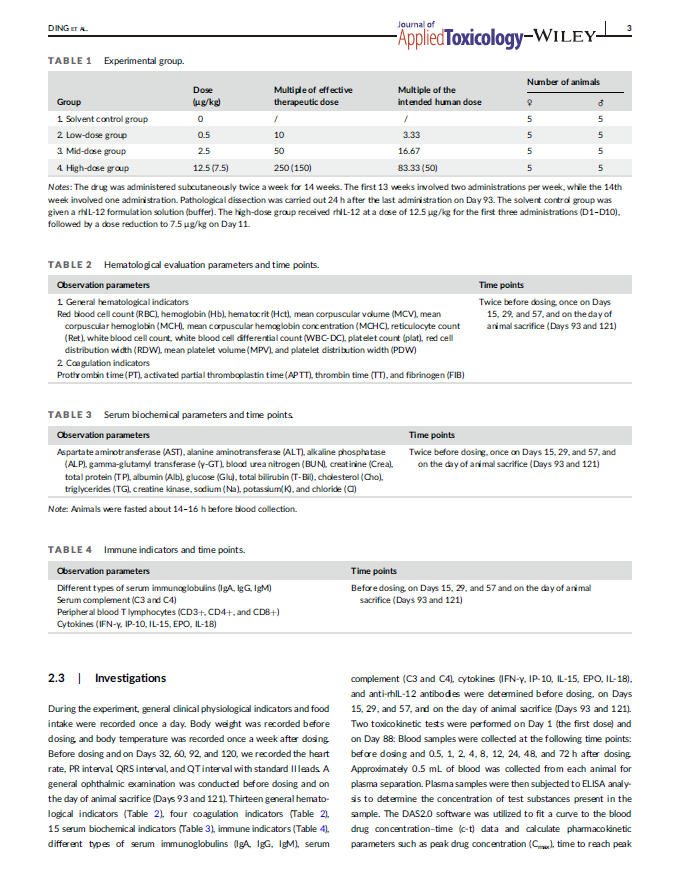
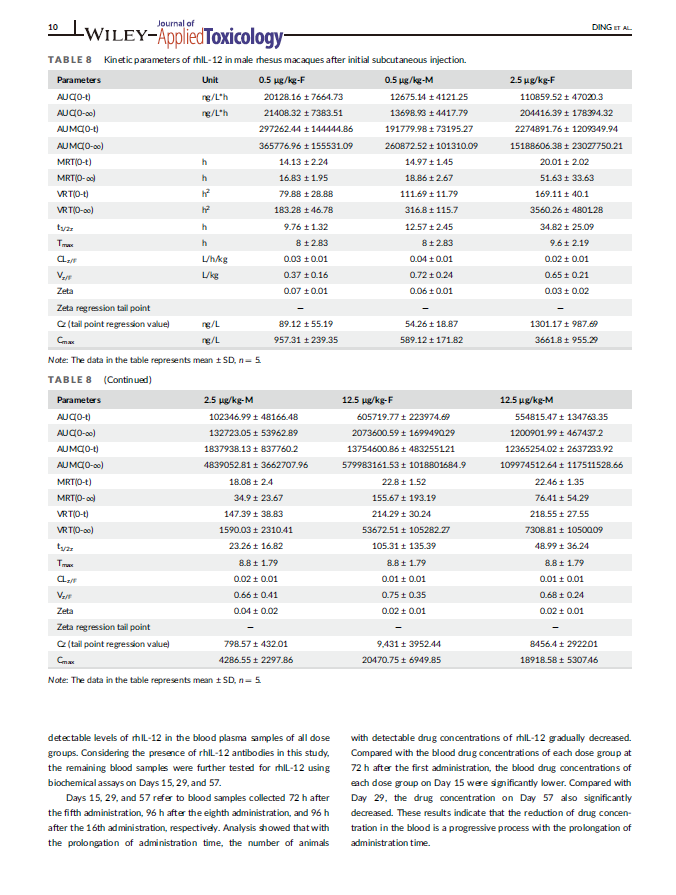
Facilitate medical research that advances human health

 1-703-853-8036
1-703-853-8036 September 28th, 2023,Dr. Quanjun Wang, CEO and CSO of SAFE Pharmaceutical Technology Co., Ltd, participated in biotechnology drug evaluation research as a corresponding author, "The repeated administration of rhIL-12 for 14 weeks in rhesusmonkeys: A toxicity assessment" . The essay was officially published in the “Journal of Applied Toxicology”, with an impact factor of 3.3.